Scintillation properties of liquified rare gases
This work has been supported by funds of the Deutsche Forschungsgemeinschaft DFG (Transregio 27: Neutrinos and Beyond) and the Maier-Leibnitz-Laboratorium (Garching).
Introduction
For the direct search for Dark Matter in our galaxy with particle detectors not only scintillating crystals (like calcium tungstate in the CRESST experiment) or semiconducting materials (like germanium and silicone in CDMS) can be used, but also liquified rare gases, essentially argon and xenon (e.g. XENON). The atoms of the rare gases get excited or ionized by the incident particle, which might be a charged particle (e.g. from a radioactive decay) exciting the atoms directly, or an electrically neutral particle (i.e. a neutron or WIMP) interacting via nuclear recoil. To a great extent, the energy deposited in the rare gas is converted into scintillation light, originating from the de-excitation and recombination of the atoms. These scintillation properties of the liquified rare gases shall be investigated with a newly built experiment, which is performed in cooperation with the group of A. Ulrich (chair E12). Hereby, the main focus is on experiments with different incident particles, namely electrons and heavy ions. The differences in the scintillation spectra of these particles may give rise to a new discrimination technique between background (radioactivity), which interacts mostly with electrons, and signal (WIMPs), which interact with the nuclei of the rare gas.
Scintillation properties and light emission
The scintillation spectrum of a rare gas shows several more or less distinct features: The direct transition of the excited atoms to the ground state
leads to the emission of the characteristic resonace lines. These lines are especially visible at low gas pressures, while for higher pressures the probability of forming excimers (excited dimers) rises:
But also during the formation of these excimers the metastable excited state can undergo a transition to the ground state and emit a photon. Nevertheless, in this case the "line" is broadened to a continuum (the so-called first continuum), as the transition energy is changing with the changing distance between the nuclei (see picture).
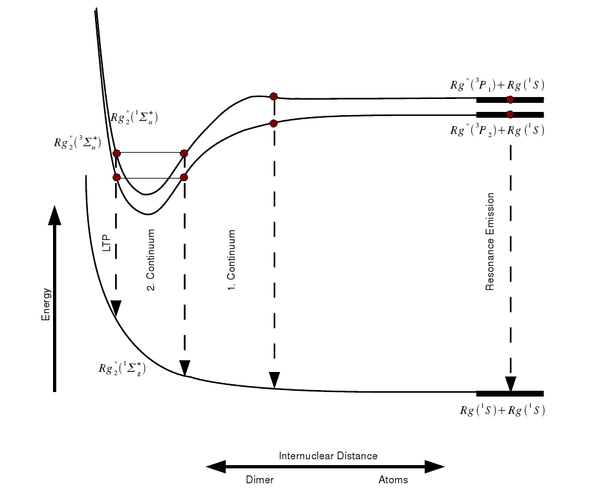 |
Therefore, the first continuum starts at the resonance lines of the rare gas and reaches up into the second continuum, which is formed by the decay of the various vibrational states of the excimers into the electronic ground state:
The second continuum is dominating the emission spectrum especially for higher pressures and excitation with electrons. Towards even longer wavelengths follows the classical left turning point (LTP), where the transition probability to the ground state is quite high.
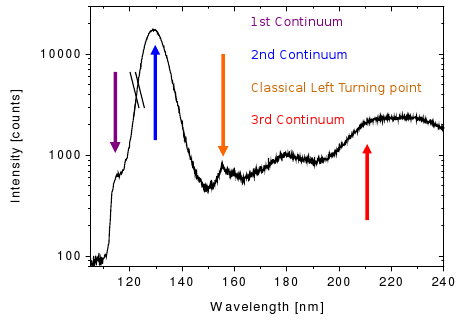 |
Not only excited atoms lead to an emission of light, but also ionized ones. Here, the scintillation processes are rather complex (see next paragraph) and give rise to the third continuum. Due to the higher energy deposition per unit track length, and thus a higher number of ionized atoms, the third continuum is mainly seen with heavy ion beam excitation of the rare gas.
Experiments with rare gases in gasous state
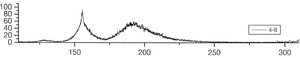
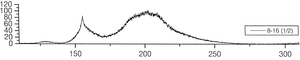
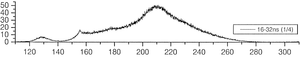
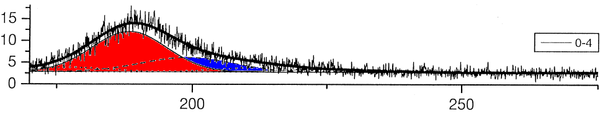
The group of A. Ulrich has already performed experiments to clarify the scintillation mechanisms of rare gases in the gasous state [1]. In the most recent one, argon was excited using a pulsed heavy ion beam at the Munich Tandem Accelerator with 2 ns pulse length, and time correlated spectra were recorded (see pictures on the right). By varying the gas pressure the single steps of the scintillation process could be intentified: The emission stemming from the first step of the gas kinetic process is found at early times and low pressures. The subsequent emissions show up at later times. Furthermore, the higher the gas pressure, the faster are the kinetic processes, hence, the very early steps can only be identified at low pressures, while the latest emission appears dominantly in the spectra recorded at high gas pressures. The single components of the third continuum, for example, were identified by applying multi-Gaussian fits to the spectra in the region of interest.
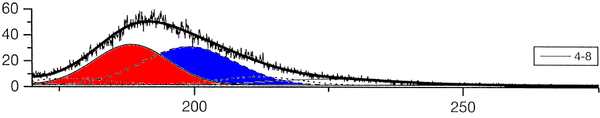
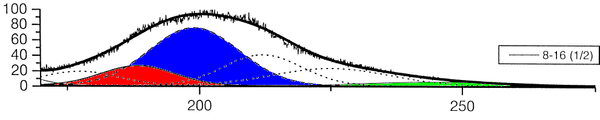
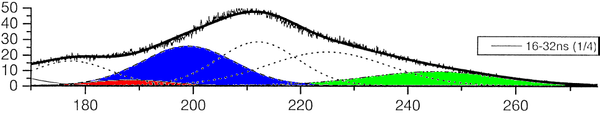 |
The peak position of one identified emission component was kept fixed in the following, allowing only for the peak amplitude (i.e. emission intensity) to vary. By depicting the peak areas as a function of the time, the time evolution, and thus the sequence of the emission processes could be determined (see picture below). The allocation of the different emission lines seen in the spectra to known emission processes finally lead to the flow diagram of species radiating.
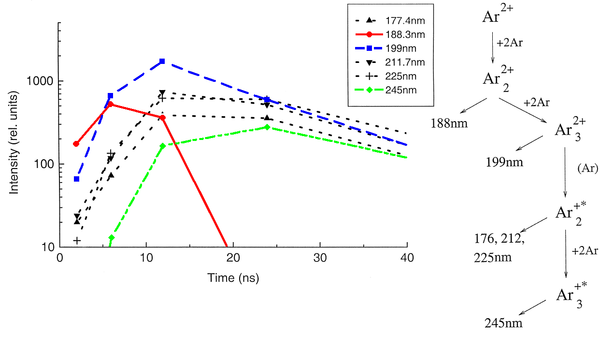
Measurements with liquified rare gases
 |
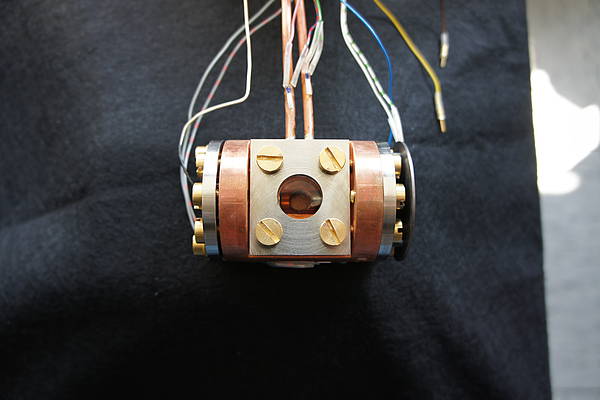
In order to be able to record time correlated spectra of the liquified rare gases both with electron and heavy ion beam excitation, two new target cells (see picture below, the target cell for the ion beam, and picture on the left: target cell for electron excitation) were designed and manufactured. Both cells are made of copper and connected by a cold finger (seen on the lefthandside) to a liquid nitrogen supply. By this, the temperature in the cell is low enough to let the heavier rare gases (argon, krypton, and xenon) condense. The temperature is monitored with several resistance thermometers and can be adjusted by switching on and off a small heater. On top, the cells are connected to a heat exchanger, which is needed, as the rare gases need to be cleaned with a gas purifier, which is working in the gasous regime only. Therefore, there needs to be a steady gas flow through the cell with continuous condensation and re-evaporation of the rare gas. The electron beam is entering the cell through a thin silicone nitride membrane, while in the case of heavy ions a thin titanium foil is used. These membranes and foils are capable of withstanding the pressure difference between the liquid inside and the vacuum outside the cell, but still thin enough to let pass electrons with energies of several keV and the heavy ions with energies up to hundreds of MeV, respectively. Furthermore, for the ion beam experiments, a tantalum aperture is connected to the cell (see picture below) in order to avoid an activation of the cell itself. The scintillation light produced in the liquified rare gas can escape from both cells through a MgF2 window, which is transparent to ultraviolett light, and is focussed with a mirror optics onto the entrance slit of the spectrometer. One focal point of this mirror is in the cell, the other one on the entrance slit. By this, a very high light collection efficiency is reached. The whole setup for the electron beam experiments and working principle is again shown in the scheme below.
 |
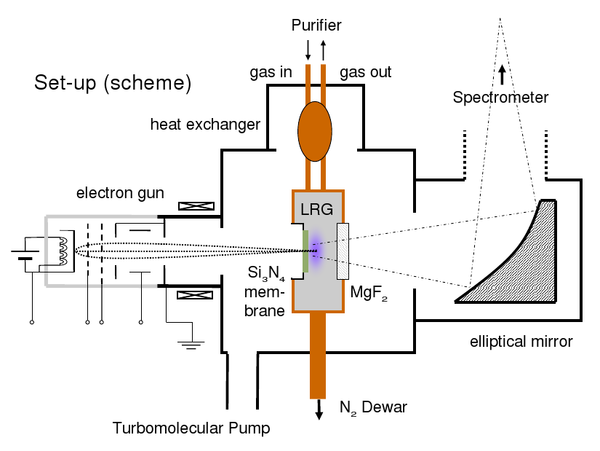

The picture on the right shows the whole setup for the electron beam experiments, where all parts have been assembled. The whitely coloured dewar contains the liquid nitrogen supply. On top of the setup the connections to the gas purifier can be seen, which is cleaning the rare gases by leting them stream over a hot titanium sponge. The turbomolecular pump on the bottom side is needed for evacuating the setup. Otherwise, the electrons from the elctron gun (covered by the cube containing the cell) could not reach the target cell. In front, the adjustable elliptical mirror can be seen, which is focussing the emitted scintillation light into the spectrometer (blue box on the righthandside). The working principle of this spectrometer is the following: A blazed grating moved by a computer controlled stepper motor is selecting the desired wavelength. The intensity at this wavelength is measured with a photomultiplier tube (the inclined part on top of the spectrometer) and recorded by the electronic measuring equipment. This equipment is able to record continuous-wave (cw) spectra, or, in combination with a pulsed particle source (electron gun or heavy ion beam) also time correlated spectra. For this purpose, a time-to-aplitude converter (TAC) is used, which converts the arrival time of the scintillation photons after the trigger (provided by the power supply of the electron gun or a beam pick-off in case of a heavy ion beam) into a logical signal of correlative height, which can be recorded using a multichannel analyser. In case of the heavy ion beam experiments, the beam intensity has to be measured, too, to be able to compare the light intensities of the various parts of the emission spectrum afterwards. By contrast, the intensity of the electron gun is stable enough for comparative measurements.

The first part of the experiments is the investigation of liquid argon with electron excitation. On the left, a picture of the light emission of liquid argon is shown (the liquid surface can be easily seen) however, a small contamination with oxygen makes the scintillation light appear green. In this first testing measurement the elliptical mirror and the spectrometer were disconnected and replaced by a glass window. Later on, also xenon shall be studied. All experiments with the electron excitation are performed in the laboratory, while the heavy ion experiments take place at the Munich Tandem Accelerator in hall II (beamline +25°).
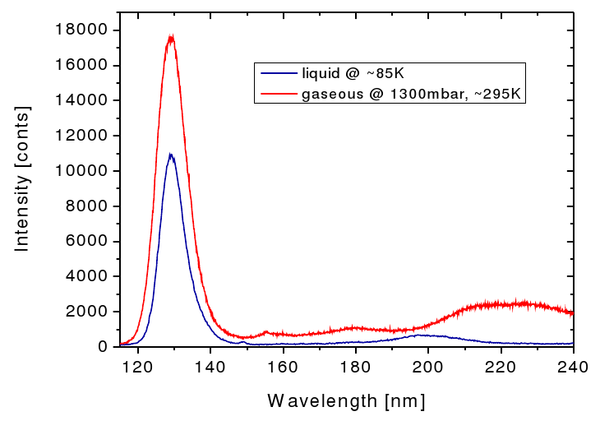
As explained above, the most intense spectral feature using electrons as incident particles is the secong continuum, stemming from the decay of the various vibrational states of the rare gas excimers. For argon this continuum is centered around ~130 nm. Just below, the emission spectra of argon in the gasous and the liquid phase, respectively, are depicted. While the secong continuum looks quite similar in both cases, some features at longer wavelengths seem to be different in the liquid phase when compared to the emission spectrum of argon gas. This needs to be addressed in an upcoming analysis.
Questions that shall be answered with the experiments:
- Which continua/lines do appear in the spectra of the liquified rare gases when using electron and ion excitation? What differences can be seen between the two excitation methods? What are the relative intensities of the different spectral features?
- How does the time structure of the distinct parts of the spectrum look like?
- What are the differences to the gasous state?
- Based on the experimental results, which kinetic processes take place and how is the scintillation light produced?
- Can the liquified rare gases be described as a dense gas or with the band model?
As an upgrade to the experiments, also admixtures of two different rare gases could be studied. Assuming for example, an argon bulk material is doped with a small amount of xenon. Then, energy can be transferred (radiatively or non-radiatively) from excited argon atoms to xenon atoms, leading to the emission of scintillation light of xenon. This is quite similar to the wavelength shifting in liquid scintillators, where a small amount of a fluor in a solvent bulk has huge effects on the emission spectrum of the admixture. Furthermore, in case of the rare gases excimers of two different species could form (e.g. Ar*Xe), which give rise to new features in the emission spectra. By studying these, one can learn more about the mechanisms of energy transfer and the kinetic processes.
Outlook on the usage of liquified rare gases as detector materials
In summary, the spectroscopic investigation of the liquified rare gases with respect to their use as detector material is meant to answer the following questions:
- Can the differences in the emission spectra, which occur when different particles excite the liquified rare gases, be used for a particle discrimination?
- How does the pulse shape look like? Again, can this be used for a particle discrimination?
- Can the wavelength shifting processes in admixtures of two liquified rare gases be used for a further improvement of the sensitivity of the detectors?
Bibliography
[1] J. Wieser, A. Ulrich, A. Fedenev, M. Salvermoser, "Novel pathways to the assignment of the third rare gas excimer continua", Opt. Comm. 173 (2000) 233-245
Publications and Talks
- T. Heindl, T. Dandl, M. Hofmann, R. Krücken, L. Oberauer, W. Potzel, J. Wieser and A. Ulrich, "The scintillation of Liquid Argon", EPL,91 (2010) 62002
-
T. Heindl, T. Dandl, A. Fedenev, M.Hofmann, R. Krücken, L. Oberauer, W. Potzel, J. Wieser and A. Ulrich, "Table-top setup for investigating the scintillation properties of liquid argon" 2011 JINST 6 P02011
Diploma and PhD theses at E15 and E12
Martin Hofmann
"Liquid Scintillators and Liquefied Rare Gases for Particle Detectors - Background-Determination in Double Chooz and Scintillation Properties of Liquid Argon", PhD thesis, Technische Universität München, 2012
Members of the liquified rare gas working group
- PD Dr. Andreas Ulrich (E12)
- Dr. Jochen Wieser (Optimare GmbH)
- Thomas Dandl (E12)
- Dr. Martin Hofmann (E15)
- Alexander Neumeier (E15)
Contact
If you are interested in a diploma or PhD thesis on this topic, please contact Prof. Dr. Lothar Oberauer or PD Dr. Andreas Ulrich.
page last edited on Feb., 27th 2011 by Martin Hofmann